Class 7 Science Curiosity Chapter 4 The World of Metals and Non-metals NCERT Solutions
Class 7 Science Curiosity Chapter 4 The World of Metals and Non-metals NCERT Solutions has been updated for the session 2025-26. These solutions cover all the questions related to the properties, differences, and uses of metals and non-metals, which are the core concepts of Chapter 4, The World of Metals and Non-metals. In addition to these topics, this chapter also discusses corrosion of metals and how metals behave in the presence of air and water.
Class 7 Science Curiosity Chapter 4 The World of Metals and Non-metals Questions Answers – PDF Download
Page 55
Let Us Enhance Our Learning
1. Which metal is commonly used to make food packaging materials as it is cheaper, and its thin sheets can be folded easily into any shape?
(i) Aluminium (ii) Copper (iii) Iron (iv) Gold
Answer:
(i) Aluminium.
2. Which of the following metal catches fire when it comes in contact with water?
(i) Copper (ii) Aluminium (iii) Zinc (iv) Sodium
Answer:
(iv) Sodium.
3. State with reason(s) whether the following statements are True [T] or False [F].
(i) Aluminium and copper are examples of non-metals used for making utensils and statues. [ ]
(ii) Metals form oxides when combined with oxygen, the solution of which turns blue litmus paper to red. [ ]
(iii) Oxygen is a non-metal essential for respiration. [ ]
(iv) Copper vessels are used for boiling water because they are good conductors of electricity. [ ]
Answer:
(i) False. Aluminium and copper are metals used to make utensils and statues.
(ii) False. Metal oxides are basic and turn red litmus paper blue.
(iii) True.
(iv) False. Copper vessels are used for boiling water because copper is a good conductor of heat.
4. Why are only a few metals suitable for making jewellery?
Answer:
Some essential properties for making jewellery include lustre, malleability, ductility, and resistance to corrosion (i.e., they don’t tarnish easily). Only a few metals, such as gold and silver, possess all of these properties, which is why they are commonly used in jewellery making.
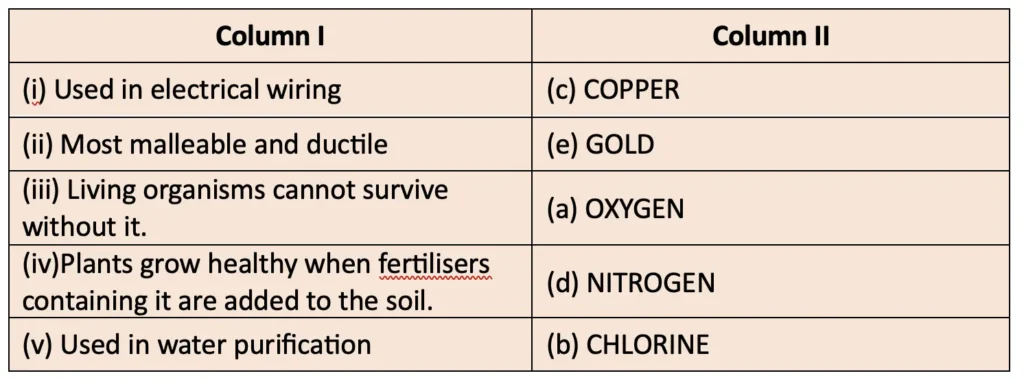
5. Match the uses of metals and non-metals given in Column I with the jumbled names of metals and non-metals given in Column II.
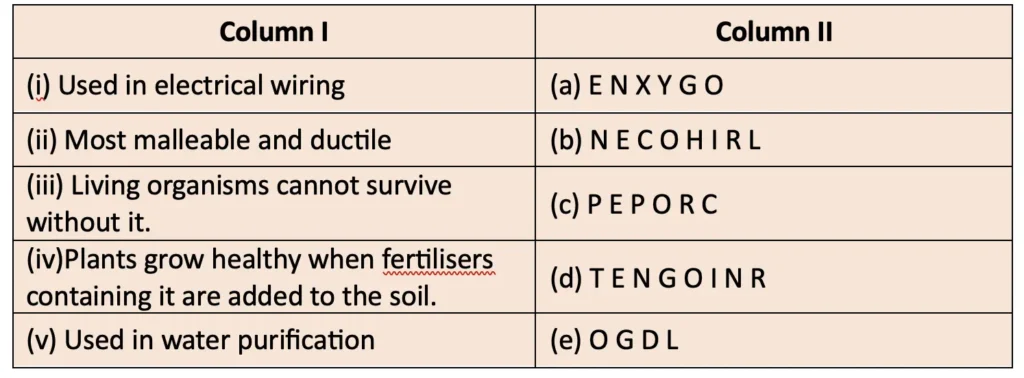
Answer:
6. What happens when oxygen reacts with magnesium and sulfur. What are the main differences in the nature of products formed?
Answer:
Magnesium reacts with oxygen to form magnesium oxide. When magnesium oxide is dissolved in water, it forms a basic solution that turns red litmus paper blue. Sulfur, when burned in air, reacts with the oxygen present to form sulfur dioxide. When sulfur dioxide is dissolved in water, it forms sulfurous acid, which is acidic in nature and turns blue litmus paper red.
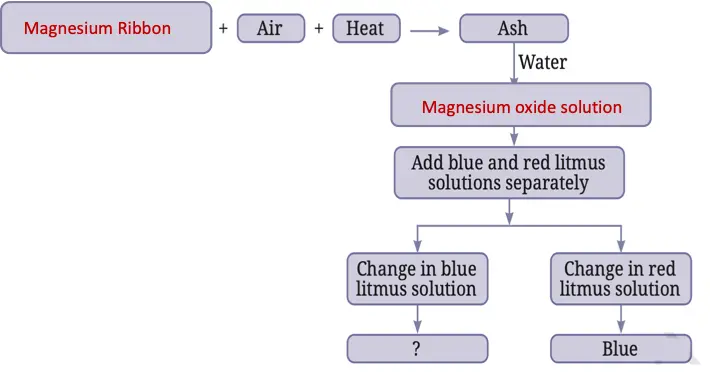
7. Complete the following flow chart:
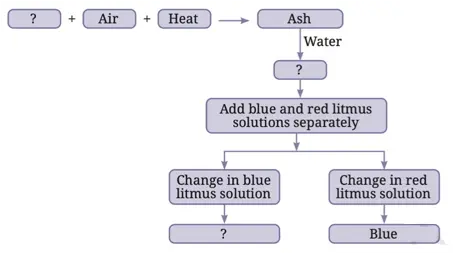
Answer:
8. You are provided with the following materials. Discuss which material would be your choice to make a pan that is most suitable for boiling water and why?
Iron, copper, sulfur, coal, plastic, wood, cardboard
Answer:
To make a pan suitable for heating, iron and copper would be the best choices. Both are metals with high melting points, so they can withstand high temperatures without melting or deforming. They are also good conductors of heat, allowing heat to spread evenly across the surface of the pan.
9. You are provided with three iron nails, each dipped in oil, water and vinegar. Which iron nail will not rust, and why?
Answer:
The iron nail dipped in oil will not rust because rusting requires the presence of both water and air (oxygen). The layer of oil prevents air and moisture from reaching the surface of the iron, thereby stopping the rusting process.
10. How do the different properties of metals and non-metals determine their uses in everyday life?
Answer:
Metals are used in making wires and cables (due to electrical conductivity); kitchenware and utensils (due to heat conductivity); structures, tools, and weapons (for their strength and hardness); bells (because of sonority); and jewellery (due to lustre, malleability, and ductility).
Non-metals are usually brittle, poor conductors, and lack metallic lustre, but they are useful in other ways—for example, oxygen for breathing, graphite in pencils, chlorine for water purification, nitrogen in fertilizers, and plastic and rubber as insulators.
11. One of the methods of protecting iron from getting rusted is to put a thin coating of zinc metal over it. Since sulfur does not react with water, can it be used for this purpose? Justify your answer.
Answer:
No, sulfur cannot be used to coat iron like zinc because it is a brittle non-metal that does not form a strong, durable, or protective layer. It can easily crack or flake off, leaving the iron exposed to air and moisture, which would allow rusting to occur.
12. An ironsmith heats iron before making tools. Why is heating necessary in this process?
Answer:
Heating is necessary in this process because it softens the iron and increases its malleability, making it easier to bend, shape, and form into the desired tool.
